
新入荷
再入荷
Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study | Journal of Clinical Oncology
 タイムセール
タイムセール
終了まで
00
00
00
999円以上お買上げで送料無料(※)
999円以上お買上げで代引き手数料無料
999円以上お買上げで代引き手数料無料
通販と店舗では販売価格や税表示が異なる場合がございます。また店頭ではすでに品切れの場合もございます。予めご了承ください。
商品詳細情報
| 管理番号 |
新品 :2568442206
中古 :2568442206-1 |
メーカー | 7d20850ca53179 | 発売日 | 2025-04-15 02:35 | 定価 | 15000円 | ||
|---|---|---|---|---|---|---|---|---|---|
| カテゴリ | |||||||||
Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study | Journal of Clinical Oncology
 Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study | Journal of Clinical Oncology,
Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study | Journal of Clinical Oncology, 798 Real-world overall survival among patients receiving first-line (1L) pembrolizumab in the treatment of recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) in the United States | Journal for ImmunoTherapy of,
798 Real-world overall survival among patients receiving first-line (1L) pembrolizumab in the treatment of recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) in the United States | Journal for ImmunoTherapy of,30690-3/asset/4b9881fe-68f8-4b34-9ede-c68740bf2c84/main.assets/gr2_lrg.jpg) Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial - The Lancet Oncology,
Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial - The Lancet Oncology,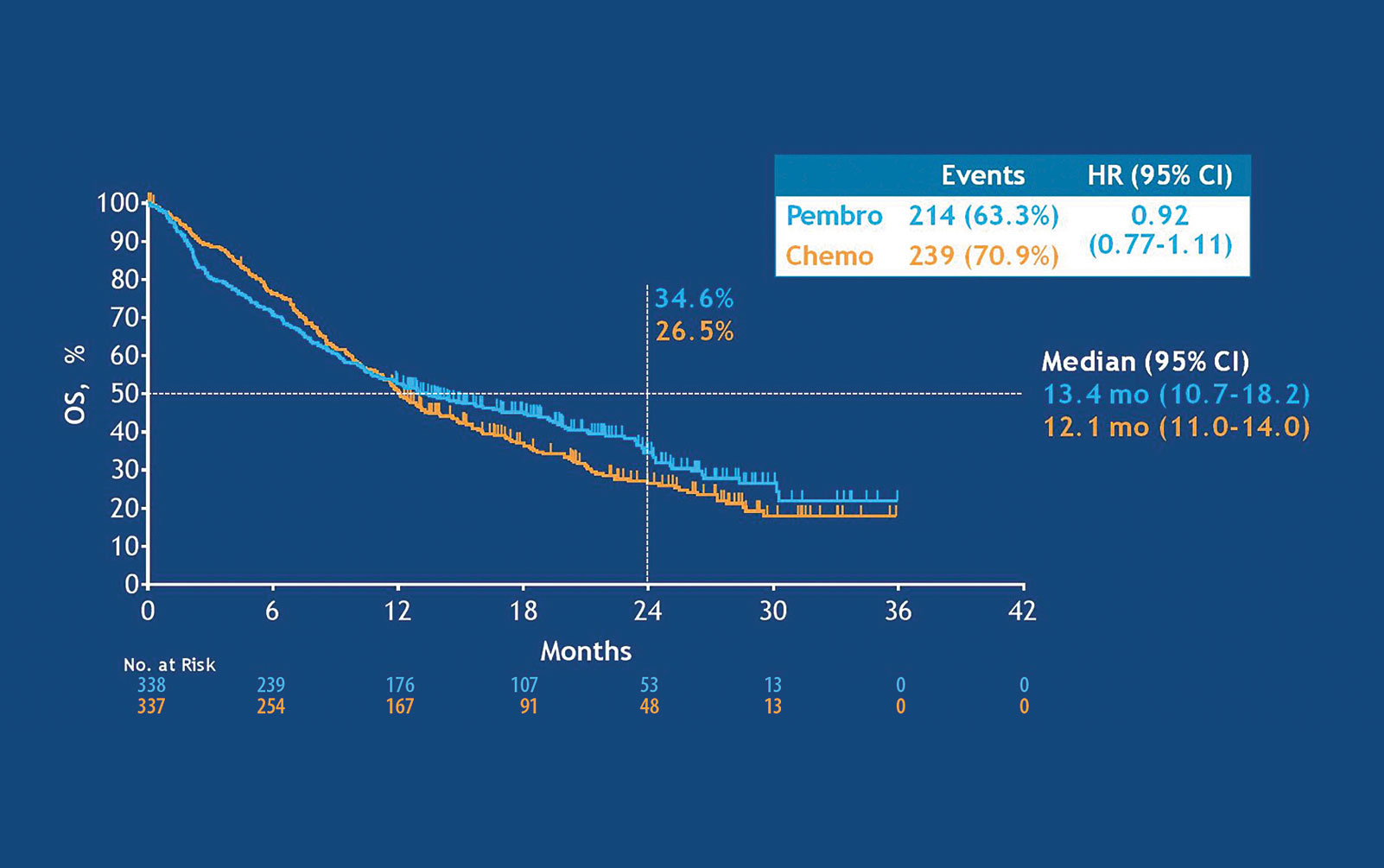 KEYNOTE-042: No Hard and Fast Rules for First-Line Pembrolizumab Regarding PD-L1–Positive Disease - ILCN.org (ILCN/WCLC),
KEYNOTE-042: No Hard and Fast Rules for First-Line Pembrolizumab Regarding PD-L1–Positive Disease - ILCN.org (ILCN/WCLC), Tralokinumab Plus Topical Corticosteroids as Needed Provides Progressive and Sustained Efficacy in Adults with Moderate-to-Severe Atopic Dermatitis Over a 32-Week Period: An ECZTRA 3 Post Hoc Analysis | American Journal of Clinical Dermatology
Tralokinumab Plus Topical Corticosteroids as Needed Provides Progressive and Sustained Efficacy in Adults with Moderate-to-Severe Atopic Dermatitis Over a 32-Week Period: An ECZTRA 3 Post Hoc Analysis | American Journal of Clinical Dermatology



























